


MANILA - The International Medical Device Regulators Forum (IMDRF) defines Software as a Medical Device as being software intended for one or more medical purposes that performs these purposes without being part of a hardware medical device. SaMD is one of three softwares related to medical devices, with the other two being ‘Software in a Medical Device’ and ‘Software used in manufacturing or maintaining a medical device.’
The IMDRF, for the purposes of harmonization, has established that software must have a valid clinical association between the output of an SaMD and the targeted clinical condition (to include pathological process or state) and the SaMD must provide the expected technical and clinical data.
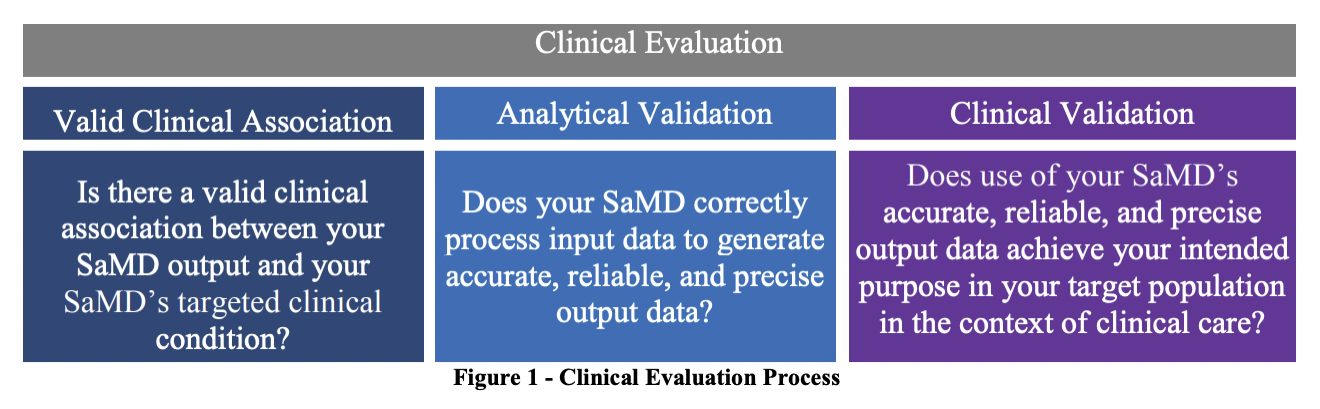
This is the exact framework that most countries have adopted and used as the basis for regulating SaMDs. However, since SaMDs can be built with artificial intelligence (AI) algorithms that learn over time, improve, and even evolve, does that imply that these applications should have their own separate category/subcategory?
Challenges in AI-based SaMDs
AI, with its vast potential, is expected to be one of the critical factors in the reform of the healthcare industry in emerging markets. The algorithms used for this software can be classed into two categories: locked or adaptive (continuous learning). In the healthcare setting, developers use adaptive algorithms to process patient data at lightning-fast speeds to discover and understand patterns and scale operations.
In the adaptive type of algorithm, the machine is presented with labeled datasets for training purposes by the developers. Since most of the SaMDs used by emerging market countries are developed overseas, the datasets u
The content herein is subject to copyright by The Yuan. All rights reserved. The content of the services is owned or licensed to The Yuan. Such content from The Yuan may be shared and reprinted but must clearly identify The Yuan as its original source. Content from a third-party copyright holder identified in the copyright notice contained in such third party’s content appearing in The Yuan must likewise be clearly labeled as such. Continue with Linkedin
Continue with Linkedin
 Continue with Google
Continue with Google









 1274 views
1274 views








